01. What is a Medical device?
In general terms, medical device is any physical entity, including apparatus, hardware, software, physical device or material, which supports humans to deal with medical circumstances.
World health organization has given a broad definition focusing on its purpose:
‘Medical device’ means any instrument, apparatus, machine or appliance for in vitro use, hardware, software, material or other related article or the combination of it used for the specific medical purposes including
- diagnosis, monitoring, prevention, treatment or alleviation of disease or an injury,
- investigation, modification, replacement, or support for a physiological process,
- supporting or sustaining life,
- disinfection of medical devices
- Providing information by means of in vitro examination derived from the human body.
02. On what basis are medical devices categorized?
Medical devices are categorized based on risk associated with usage of the device. The categorization is based on the following parameters:
- The general regulatory controls
- The special regulatory controls
- The need of premarket approval (PMA)
What are general controls for medical devices?
General controls are fundamental provisions to ensure the safety and effectiveness of regulatory devices. It includes provision for adulteration, misbranding, device registration, notifications for repair/replacement or refund, premarket notification, records & reports, and good manufacturing practices.
What are special controls for medical devices?
Special controls are additional controls to general controls for reasonable safety and usability assurance, which includes specific labeling such as usage methods, guidelines, recommendation etc.; performance standards; post-market monitoring; patient registration and more.
03. What is premarket notification?
Premarket notification, also known as 510(k) notification is mandatory registration for manufacturers to notify FDA (Food and Drug Administration) about their intent to market a medical device.
For any manufacturer, before they start marketing a medical device in United States, its mandatory to prove to FDA that it is subsequently equivalent to a device already available in the market.
510(k) notification is to demonstrate that the medical device is as safe and effective as other equivalent devices legally available in the market, and that is not subject to premarket approval.
Until the applicant receives an order that declares the device as safe and effective, the applicant must not market the device.
04. What is Premarket Approval (PMA)?
PMA is a process brought in practice by FDA (Food and Drug Administration) to review a medical device for safety and effectiveness evaluation. For class III medical devices, there is high level of risk associated. FDA forces PMA under section 515 of the FD&C Act to assure the safety before bringing any device to market. PMA application is an instrument to provide a valid evidence to prove that device is safe and effective for the claimed use by collecting data from human clinical trials.
If a manufacturer wishes to market a new kind of device, which is not available in market or having a different material or different design than previously available devices, to ensure the primary safeguard of user, manufacturer must submit a premarket approval application to the FDA for review.
A manufacturer must receive approval in response to PMA application before they start to market a device. FDA authorities provide a time of 180 days to make an approval decision, but generally, it takes more time before approving or denying the premarket approval application.
Read the this article to learn more about 510(k) notification, PMA, and other FDA forced medical device regulations.
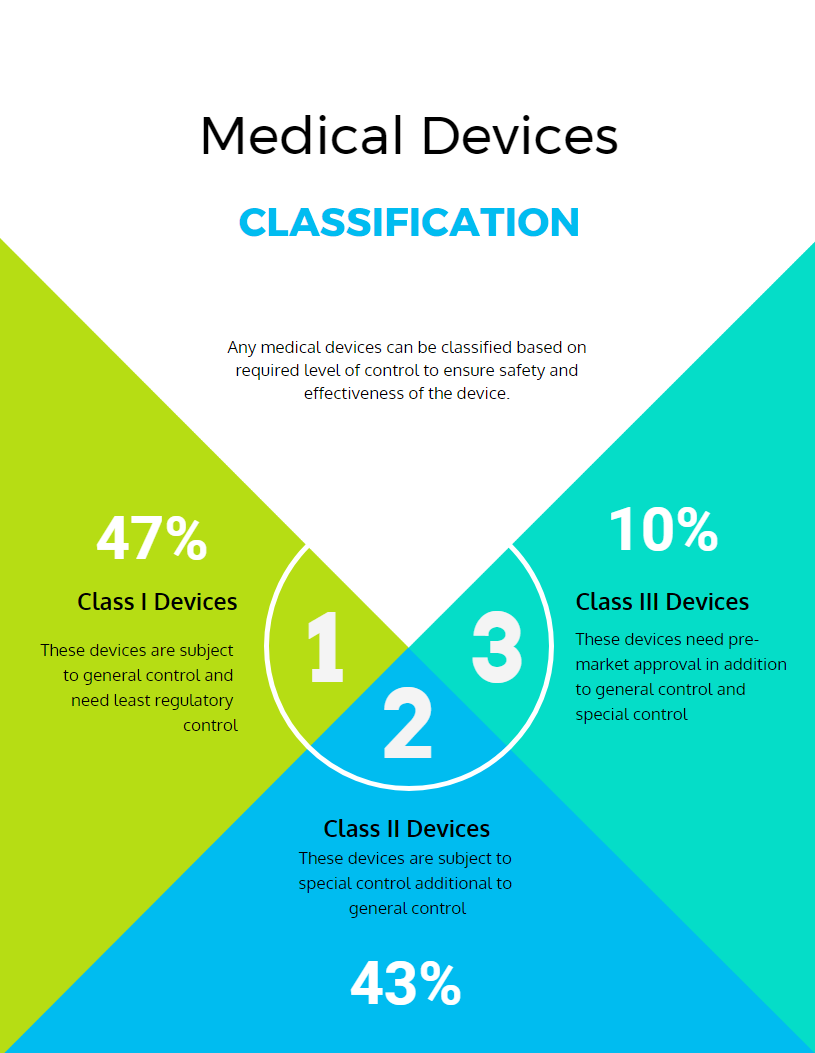
05. How Medical devices can be classified?
Medical devices can be classified based on required level of control to ensure safety and effectiveness of the device. As this is risk-based classification, the major class determination factor is the risk the device poses to the patient or the user.
According to FDA, Medical devices can be classified into three classes: Class I (General controls), Class II (General controls with special controls) and Class III (General controls, Special Controls and premarket approval) devices.
Class I: These devices are subject to general control and need the least regulatory control. This class of devices have the lowest level of risks. Class I devices are not planned to help, support or sustain life and may not present any irrational risks of illness or injury to the user. Most of these devices do not need premarket notification and some exempt to follow manufacturing practice regulation.
Examples: bandages, hand-held surgical instrument, examination gloves, etc.
Class II: These devices are subject to special controls, in additional to general control. Special controls are device specific regulatory controls that ensure that the devices meet the intended functionality. The devices for which general control alone cannot assure safety and effectiveness, will require additive control similar to that of the existing approved devices. Few of these devices are exempted from premarket notification and requires special labeling, performance standards, and post market observation as special controls.
Examples: acupuncture needles, powered wheelchairs, infusion pumps, pregnancy kits, and surgical drapes, etc.
Class III: Devices for which even special controls in addition to general control cannot provide sufficient assurance for safety and effectiveness, and which needs premarket approval for a review concerning safety and effectiveness will fall under this class. The aim of these devices are to support and sustain human life functions.
Examples: implantable pacemaker, pulse generators, HIV diagnostic tests, and breast implants, etc.
06. What is IDE?
Investigational Device Exemption (IDE) is a provision that allows manufacturers to collect device specific safety and effectiveness data of proposed device before commercialization, which can be used to support premarket approval application or in some cases for premarket notification submission.
Safety and effectiveness data is the usage data collected by monitoring a provisioned device about safety and the performance according to proposed intent.
For 510(k) notification, FDA needs clinical data to provide marketing clearance in very limited cases. An approved IDE application provides rights to lawfully ship the devices for investigation purpose without forcing other FDA needs that required for device commercialization.
A manufacturer must submit complete IDE application to FDA. Although, it does not have pre-determined format but they are bound to imply certain information in the form, such as sponsor investigator information, clinical plan overview, investigational plan method & controls information, and more.
07. What is Humanitarian Use Device (HUD)?
Humanitarian Use Device program came into existence in 1990 under the Safe Medical Device Act. This program allows getting market approval for medical devices that deliver benefits to patients in treatment and diagnosis of rare diseases or medical conditions that impact fewer than 4000 individuals in the United States per year.
In December 2016, the population benchmark was changed from fewer than 4000 individuals to not more than 8000 individuals to qualify for the HUD program under 21st Century Cures Act, section 3052.
To obtain an HUD approval for a rare condition medical device, an applicant must prove that the device meets the definition, and also provide relevant reference documents describing disease or condition that the device can address, proposed usage, indications and a reason for the need of developing such a medical device.
08. What is a Humanitarian Device Exemption (HDE)?
Humanitarian Device Exemption is an approval process for HUDs for marketing device without requiring evidence of effectiveness. The application for HDE is very similar to Premarket Approval (PMA) application.
Since an HUD is exempted from the effectiveness requirements (unlike PMA application), an HDE application can be filled without the results of scientifically valid clinical investigations that demonstrate the device is effective and safe for proposed condition or disease. But it must contain the facts that the device has more health benefits than the associated risk of illness and injury compared to available devices or treatments.
Upon approval of such applications, the applicant is authorized to market a Humanitarian Use Device subject to certain profit and usage restrictions.
09. What are the regulations that manufacturer must comply in the United States?
Center for Devices and Radiological Health regulates the firms involved in manufacturing, repackaging, labelling or importing of medical devices in the United States.
Below are the basic regulatory requirements that distributors must comply with in order to get an approval from FDA:
- Establishment Registration – 21 CFR Part 807
- Medical Device Listing – 21CFR Part 807
- Premarket Notification 510(k) – 21 CFR Part 807 Subpart E
- Premarket Approval (PMA) – 21 CFR Part 814
- Investigational Device Exemption (IDE) – 21CFR Part 812
- Quality System Regulation (QS)/Good Manufacturing Practices (GMP) – 21 CFR Part 820
- Labeling – 21 CFR Part 801
- Medical Device Reporting – 21 CFR Part 803
10. What is Design Controls in Medical Devices?
Design controls is a formal methodology to drive medical product development activities, which are often mandatory. Medical device manufacturers must follow design control requirements under 21 CFR Part 820.30 to market all class 2 and 3 devices as well as certain class 1 devices such as software controlled devices.
Design control is a set of quality practices and procedures that control the design process to assure that the device meets the user needs, intended uses and specified requirements as well as improve and prevent future issues.
On the highest level, design controls requires Medical device design & development planning, design input, design output, design review, design transfer, design changes and design history file.
HANDPICKED CONTENT
Implementing Design Controls for Medical Devices with a Concurrent Engineering Approach
11. What is Design Input in Design Controls?
Design inputs are fundamentals of medical devices. Design inputs are design requirements to address the proposed uses as extracted from users’ or patients’ needs.
It is mandatory to document, review and approve the design input requirements. These inputs may include:
- Functional and performance requirements, according to the proposed use
- Safety requirements
- Applicable regulatory requirements
- Information derived from similar historic designs, where applicable
- Other requirements essential for design and development
- Outputs of risk management
Design Requirements, Design Input Requirements, Design & Development Requirements, Product Requirements can be interchangeably used with the term Design Inputs.
12. What is Design Output in Design Controls?
Design output is the result derived from each step of design process efforts as well as at the end of entire design process effort. A complete design process output will have the device, packaging and labeling information along with the device master record.
Design output provides a strong description of all the ingredients of a device including drawings, components, parts, materials, specifications, pieces, manufacturing instructions, and inspection procedures.
FDA mandates to establish and maintain processes for defining and documenting design output so that it allows an adequate evaluation of design input requirements for manufacturers under FDA 820.30(d).
13. What is Design Verification in Medical Device Development?
Design verification is a qualification testing methodology that ensures that a medical device is designed as it is intended.
Verification is an internal process, which evaluates whether a design output meets the specified requirements, specification or regulation defined in the design input.
Verification testing may occur at any point in the process, beginning from the ideation & conceptualization phase to the post-production phase.
14. What is Design Validation in Med Device Development?
Design validation is a testing from the market perspective. It is intended to validate whether a medical device is meeting the user’s needs.
Successful validation proves that a device meets needs of users in the targeted market. Although validation comes into the picture at a later stage of product development process, it still measures the very first part of the complete process, which is defining user needs.
Validation must involve testing and clinical evaluation. Also, it requires a device to be developed in the production environment with an involvement of end-user for actual or simulated testing.
15. Why is Design Verification and Validation needed?
Medical device users look for effectiveness and safety of devices that they use to address a particular problem or condition, which are sometimes critical to life. This is why iterative testing with verification and validation of these medical devices becomes imperative.
Verification and validation of medical devices in the design process aim to ensure that the device is aligned with the need of targeted users and delivers the intended solution. It also helps ensure whether all the requirements are being satisfied. It helps to comply with regulations as well as designing the highest quality product.
Standardized verification and validation activities can also streamline the manufacturing process as well as enhance approval process.
16. What is Design History File?
Design history file (DHF) is a mandatory documentation you create throughout the medical device design and development process. It is mandatory for each device class.
DHF should contain all the records necessary to validate that the device design was developed according to the accepted design plan. In case if it does not contain similar information, then it must provide a reference to that information.
It must contain all the records starting from the user needs, design input, design output, design changes, verification & validation methods & reports, design review and design transfer information.
17. What is Design Review in Medical Device Development?
Design reviews are defined checkpoints throughout the medical device development process with an aim to ensure safety and effectiveness of the device.
It also makes sure that each step of design controls is captured and documented during the product design phase.
FDA asks manufacturers to establish and maintain procedures that ensure design review documentation. To facilitate the procedures, they should pre-define appropriate stages in the medical product development process for the design review.
The procedure requires to have three types of individual(s) during each design review stage:
- Individuals or representatives of all functions concerned with the particular design stage being reviewed
- An individual(s) who does not have direct responsibility for the design stage being reviewed
- Any relevant specialists for the concerned stage, if needed.
The results of the design review should be documented in the design history file, which includes details such as identification of the design, the date, and the individual(s) performing the review.
18. What is Design Change in Medical Device Development?
Design controls specify requirements for design changes under section 820.30 (i) Design Changes:
“Each manufacturer shall establish and maintain procedures for the identification, documentation, validation or—where appropriate—verification, review, and approval of design changes before their implementation.”
This section provides guidance for controlling changes and modifications in the design of the device during the product development phase with an aim to ensure that design requirements are met before transferring to the production phase. These changes and updates come under design change procedure.
The design changes may happen early in the design process (pre-production changes) or after design being transferred to production (and post-production design changes). But early changes are relatively less expensive. The pre-production changes can happen in the conceptualization or ideation stage, prototype stage or testing stage.
19. What is Design Transfer in Medical Device Development?
Design transfer is to make sure that the design is correctly transferred into production specifications for manufacturing of the medical device. In fact, it is a confirmation that final product will be manufactured as per the intention of the design team.
Generally, it requires a review of the DMR (Device Master Record), which is a master design documentation for building the device. Review ensures that all the needed drawings are available and the production specifications are in sync with the design. It is a final check on any of the critical dimensions that are a must.
20. What is a Quality System?
A quality system is a system of processes and procedures that an organization implements to describe how the company addresses Design Controls requirements and other medical device regulations.
The FDA enforces to establish a quality system under 21 CFR Part 820, which you must follow if you are planning to go to market in the USA.
In Europe, it requires establishing a quality system to meet the medical device guidelines either under ISO 13485 or EN ISO 13485. For Canada, it validates quality system certified by ISO 13485.
FDA 21 CFR Part 820 and ISO 13485 are similar, which means you can establish a quality system that fills requirements for the USA, Canada, and Europe.
21. What is the difference between Product Development and Design Controls?
In a broader term, product development and design controls are very similar.
Product development is about developing a product while design control is about ensuring to develop the “right” product. Apparently, one can say that Design Controls are a part of a broad product development process.
The product development process includes developing some prototypes, test planning, feedback mechanism, etc. It aims to reach the launch phase of the medical device. On the other hand, design controls are all about ensuring the safety of the medical device, making sure that a medical device meets the defined requirements; the user needs and the product’s intended use, and manufacturability of the designed and developed device.
22. What is Medical Imaging?
Medical imaging is the field of healthcare involving technology, techniques, and processes to create visual representations of the internal body or the function of some organs or tissues for clinical analysis and medical intervention.
Medical imaging reveals the body structure hidden behind skins and bones to diagnose and treat diseases. It identifies abnormal structures compared to an established database of normal anatomy and structures.
23. Are Radiology and Medical Imaging same?
Although both radiology and medical imaging are perceived same, there is a little difference between them. Both are part of a wider discipline of biological imaging, which aims to incorporate both the fields to diagnose and treat diseases.
Medical imaging involves imaging techniques such as X-Ray, MRI and more to get the image of internal body structures. On the other hand, radiology is the field to read and interpret those images for diagnosis and treatment.
24. What are the currently used Medical Imaging techniques?
Common types of imaging techniques in use are:
- X-rays
- CT scan (computed tomography scan)
- MRI (magnetic resonance imaging)
- Ultrasound
- Nuclear medicine imaging
Each of these techniques uses a specific technology. The use of techniques depends on the need as every technique is different in terms of the amount of details it delivers. For example, X-rays are best at finding a bone-break.
25. What is a Telemedicine?
Unlike traditional healthcare setup, telemedicine does not require the physical availability of the patient and doctor. Telemedicine uses information technology and telecommunication to deliver care regardless of distance and time.
These technologies facilitate communications between patients and care givers with both convenience and reliability.
Here is a quick example of telemedicine: A mobile app that lets physicians treat their patients remotely via video capability.
26. What is an EHR?
An electronic health record (EHR) is an electronic/digital copy of a person’s health data. EHRs are stored mostly on cloud platforms and involves patient-centric health data that can be accessed by an authorized user using an electronic device instantly and securely.
It not only contains the medical and treatment histories of patients, but also aims to go beyond standard clinical data collection practices and represents a broader view of a patient’s care.
Information that an EHR can provide includes a patient’s medical histories, diagnosis, medications, treatment plans, immunization dates, allergies, radiology images, and laboratory and test results. It allows doctors to access the evidence-based tools that can be used to make decisions about a patient’s diseases and treatments, automating and streamlining the care delivery workflow.












