The international standard for medical device quality management systems, ISO 13485, offers guidelines and procedures to ensure that medical device manufacturers meet the highest standards of safety and effectiveness for their products. Compliance with ISO 13485 establishes a framework for companies in the medical device industry, covering aspects such as development, production, installation, servicing, and distribution of medical devices. It is essential for companies to demonstrate effective implementation of processes that ensure product safety and effectiveness throughout the entire product life cycle. Adhering to these guidelines enables companies to protect their customers from any potential harm associated with their products or services.
Medical device design companies need to adhere to the ISO 13485 guidelines to ensure the safety of their devices for the following reasons
- Risk Management: Medical devices have the potential to cause harm if not designed, manufactured, and distributed safely and effectively. By adhering to these guidelines, medical device design companies can identify potential hazards and implement measures to mitigate or eliminate them, thereby reducing the risk of harm to patients and users. Following ISO 13485 also helps medical device manufacturers maintain their competitive edge by providing safe and effective products.
- Quality Control: ISO 13485 requires medical device design companies to establish a quality management system that ensures consistency and reliability in the design, manufacture, and distribution of medical devices. By complying with this standard, companies can implement processes and procedures to monitor and control the quality of their devices, ensuring that they meet the required specifications and perform as intended.
- Customer Satisfaction: Medical device design companies are required to demonstrate their commitment to meeting customer needs and expectations, as outlined by ISO 13485, to ensure customer satisfaction. By adhering to this standard, companies can ensure that their devices are designed to meet the needs of patients and healthcare providers, and that they are safe, effective, and user-friendly. Following ISO 13485 helps companies prioritize customer satisfaction and deliver high-quality products that meet their customers’ requirements.
ISO 13485 Compliance Guidelines
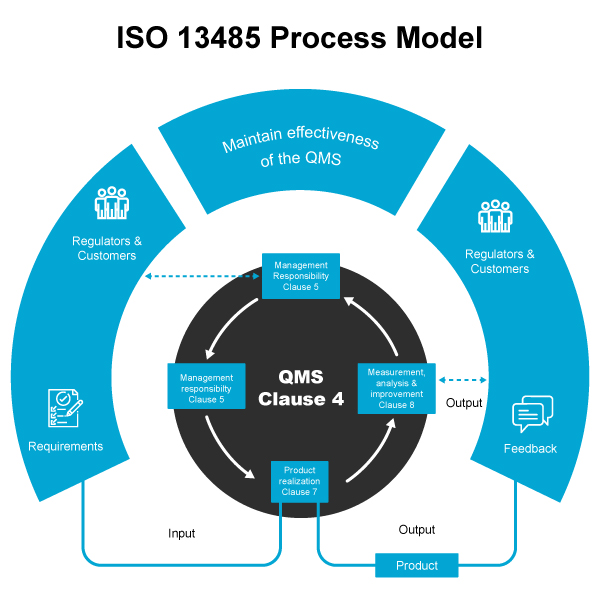
- Documentation: The standard mandates that medical device manufacturers maintain comprehensive documentation, including procedures and records, to demonstrate compliance with ISO 13485. This documentation should encompass all aspects of the Quality Management System (QMS), such as policies, procedures, work instructions, and records.
- Management Responsibility: The standard requires top management of medical device manufacturers to exhibit their commitment to quality by establishing and communicating a quality policy, ensuring the implementation and maintenance of the QMS, and providing necessary resources for its successful operation.
- Resource Management: The standard necessitates medical device manufacturers to allocate the required resources, including personnel, infrastructure, and equipment, to effectively implement and maintain the QMS.
- Product Realization: Medical device manufacturers must establish processes for product realization, encompassing design and development, procurement, production, and servicing, as outlined by the standard.
- Measurement, Analysis, and Improvement: Medical device manufacturers are obligated to establish processes for monitoring and measuring the effectiveness of the QMS, which includes assessing customer satisfaction, conducting internal audits, and conducting management reviews.
Best Practices for ISO 13485 Documentation
Define Your Risk Management Plan
The risk management plan is a document that outlines how a company will handle the risks associated with its ISO 13485 compliance process.
The risk management plan is structured into several sections, which include:
- The business environment and objectives of ISO 13485.
- The current and potential threats to the company.
- The company’s policies, procedures, and processes are to be used to effectively manage these risks.
- A description of how the company will keep track of its progress towards achieving ISO 13485 compliance.
- A detailed description of how the company will report on risks, particularly those identified as high priority by auditors during audit checks.
Use A High-quality Document Control System
The standard facilitates planning, implementing, and maintaining an effective quality management system. It covers the processes of product development, manufacturing, distribution, and customer service.
Ensuring ISO 13485 compliance with a high-quality document control system guarantees easy access for employees involved in the project to documents stored in an organized manner. It ensures easy sharing of important documents between divisions or departments by implementing a document control system for ISO 13485 compliance. This prevents any single person from having access to all relevant information.
Clear And Unambiguous Documentation
- Familiarize yourself with the ISO 13485 standard and its documentation requirements.
- Use clear and concise language when documenting processes and procedures. Avoid technical jargon and acronyms that may not be understood by everyone.
- Organize your documentation in a logical manner, with clear headings and subheadings. Use tables, flowcharts, and diagrams where appropriate, to clarify complex processes.
- Provide context for each document by explaining why it is important and how it fits into the overall quality management system.
- Review your documentation regularly to ensure it remains up-to-date and accurate. Revise and update documents as necessary to reflect changes in processes, procedures, or regulations.
Production And Servicing of Devices – Resource Authentication
- Identify the specific resources required for production and servicing of devices. This may include equipment, materials, personnel, and facilities.
- Develop procedures for acquiring, maintaining, and verifying the resources needed for production and servicing of devices. These procedures should cover the entire lifecycle of the resources, from selection and acquisition to retirement or replacement.
- Document all the processes for resource management as ISO 13485 requirements. This documentation should be clear, concise, and easy to understand, with clear links to the relevant clauses of the standard.
- Ensure that your staff is trained in the procedures for resource management, including resource identification, acquisition, and verification of their effectiveness.
- Conduct regular audits of your resource management processes to ensure that they are effective and compliant with ISO 13485 requirements. Address any non-conformities identified during these audits promptly and thoroughly.
- Continually improve your resource management processes by analyzing data, monitoring performance metrics, and identifying areas for improvement.
Bottomline
Compliance with ISO 13485 guidelines is crucial for medical device design companies to ensure the safety, effectiveness, and regulatory alignment of their products. By managing risk, controlling quality, and meeting customer expectations, companies can protect patients and healthcare professionals while improving product reliability. ISO 13485 compliance fosters trust, reduces recalls, and enhances patient safety.
Here’s how we assisted our client in developing a FDA Class II telehealth examination and diagnosis device with the following services:
- Design compliant with ISO 13485 and IEC 62304
- Development of a portable home examination modular device based on Qualcomm® Snapdragon™ 410
- Dual camera image tuning for adapters and extensions of the telehealth device.












