ISO 13485 Compliance: Guidelines and Benefits for Medical Device Design

ISO 13485 is the global standard for medical device quality management systems, ensuring adherence to rigorous safety and effectiveness standards. It provides comprehensive guidelines for all stages of medical device development, production, installation, servicing, and distribution. Compliance with ISO 13485 is crucial to demonstrate a company’s commitment to product safety and effectiveness throughout the entire product life cycle.
Understanding IEC 62304 in Medical Device Software Development
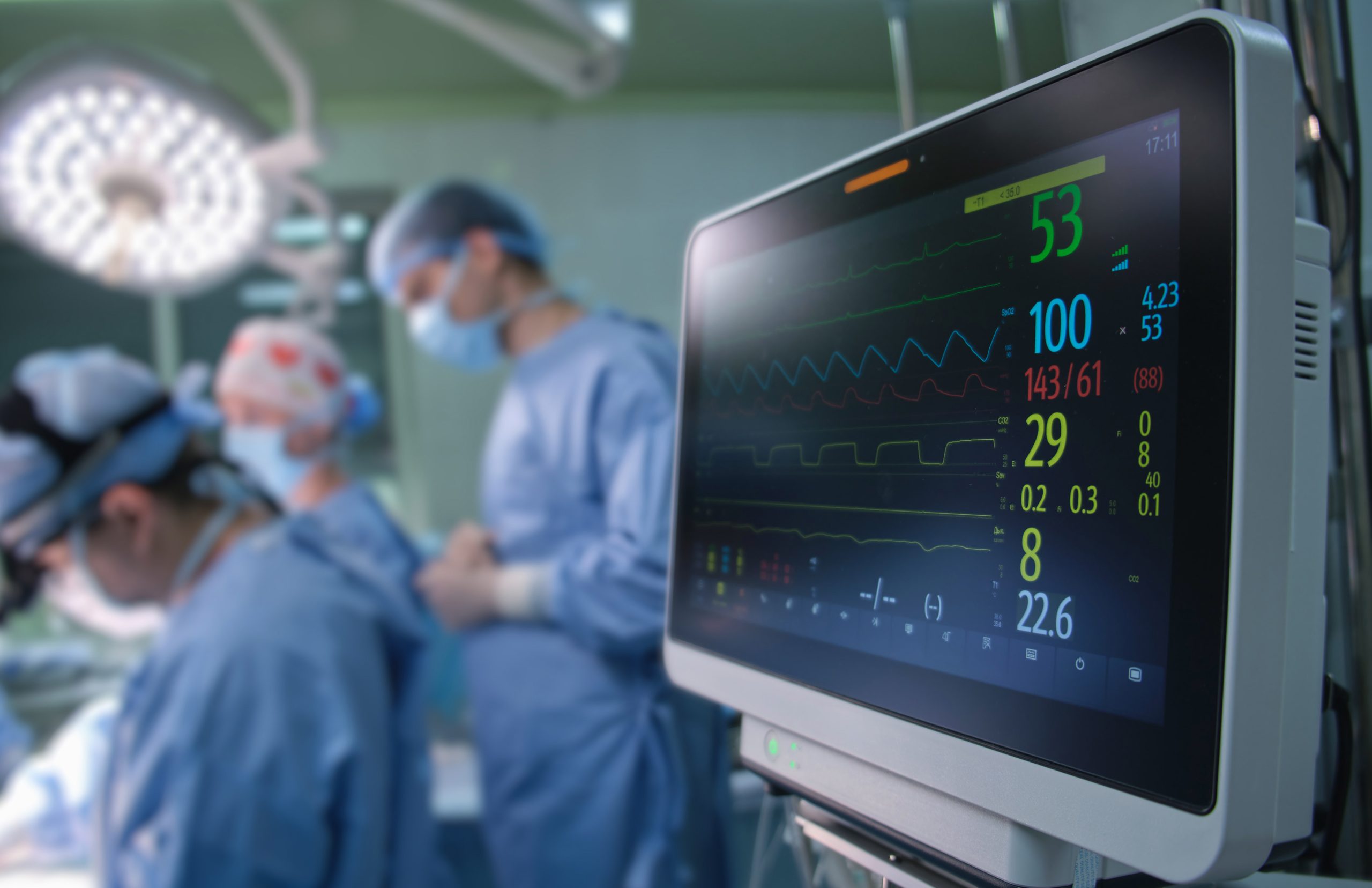
The International Electrotechnical Commission (IEC) 62304 standard outlines the requirements for the development, maintenance, and lifecycle management of software used in medical devices. Adhering to IEC 62304 is critical for medical device developers to ensure that their products meet safety and performance standards. The blog explains the risk-based approach to software development and the need for a comprehensive software roadmap to ensure durability, functionality, and compliance. The guidelines for utilizing IEC 62304 in medical device development are also discussed, emphasizing the importance of practicing due diligence to maintain the highest level of compliance with safety standards.
Revolutionizing MedTech with Robot-Assisted Surgery

Robotics has revolutionized the MedTech Industry by assisting surgeons in performing surgeries with computer-controlled robots. These advancements leverage an accurate, automated surgery system to tailor surgical procedures to the patient’s disease. Minimally invasive surgery can be performed alone or in conjunction with traditional open surgery, depending on the situation.
Operation Room Integration: The Future of Healthcare
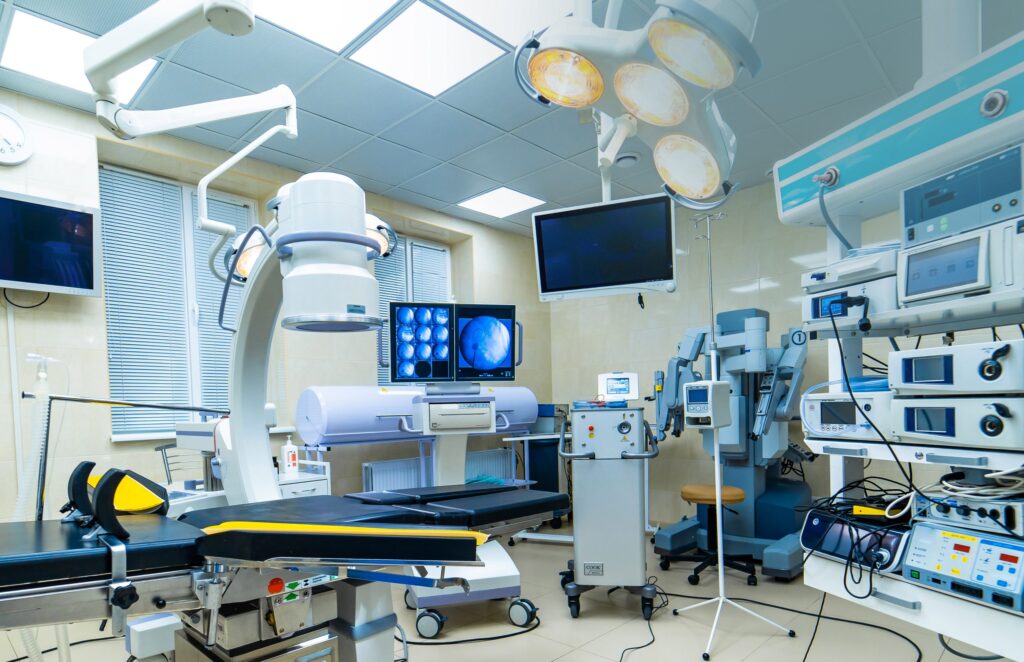
OR integration enables faster data sharing between departments, improving patient care and efficiency. Key drivers of the market’s growth include demand for advanced medical equipment and adoption of minimally invasive surgeries. Integration of wireless tech and cloud connectivity transforms OR equipment use, enabling smoother communication and information transfer. This drives adoption of OR integration systems to improve patient outcomes and reduce costs in healthcare.
Revolutionizing Diagnostics: The Rise of Non-Invasive Solutions

Recent advancements in non-invasive diagnostics, including real-time biometric monitoring, enable healthcare providers to detect diseases without relying on invasive techniques like blood tests. By utilizing sensors and algorithms, diseases can be predicted and prevented through faster early detection.
Big Data Analytics in Healthcare Services: The Revolution

The healthcare sector is being revolutionized by big data, which includes hospital data, test results, and patient records. Big Data in Healthcare can provide professionals with actionable insights that can improve services, reduce health risks and costs.
Top Medical Device Trends for 2024 and Beyond

Innovation in the medical technology industry is booming like never before, positioning 2024 as a promising year for the sector. After the pandemic, the medical devices industry has increased investment in the innovation and implementation of modern technologies such as Artificial Intelligence & Machine Learning, Augmented & Virtual Reality, the Internet of Things (IoT), Robotics, and Data Management Methodologies. This has led to the development of devices such as 3D printers for tissue regeneration, robots for surgical assistance, and advanced medical imaging equipment for improved healthcare diagnostics, among other types of medical devices.
Bring Internet of Medical Things (IoMT) Devices to Life with Qualcomm® QCS8250 and QRB5165

Connected technologies, advances in chip design, and AI at the edge are helping the healthcare industry in innovative ways. AI-enabled and 5G-driven Internet of Medical Things Devices (IoMT) backed by Qualcomm’s small-form-factor platforms – QCS8250 and QRB5165 – can do wonders for developing future-looking healthcare applications as these platforms support multiple camera streams, 5G, WiFi6, Bluetooth 5.1, and AI at the edge with heterogeneous low-power compute.





